Liste von anorganischen Verbindungen
Die folgende Liste zeigt eine Auswahl anorganischer Verbindungen. Die Liste erhebt keinen Anspruch auf Vollständigkeit. Im ersten Teil werden die Salze der Elemente nach jeweils aufsteigender Molmasse der wichtigsten Zweielementverbindungen und Sauerstoffsäuren aufgelistet.
Die Tabelle weiter unten listet umgangssprachliche Namen (Trivialnamen) mit den entsprechenden systematischen Namen (IUPAC-Namen) bekannter anorganischer Verbindungen alphabetisch auf.
Anorganische Verbindungen aus zwei Elementen
Folgende Übersicht gibt einen Überblick über anorganische Verbindungen, welche aus zwei verschiedenen Elementen bestehen.
Hydride
Salzartige Hydride enthalten negativ geladene Wasserstoffionen, die Hydridionen.
| Element (Kation) | Oxidationszustand | Hydrid |
|---|---|---|
| Lithium | +1 | Lithiumhydrid (LiH) |
| Beryllium | +2 | Berylliumhydrid (BeH2) |
| Natrium | +1 | Natriumhydrid (NaH) |
| Magnesium | +2 | Magnesiumhydrid (MgH2) |
| Aluminium | +3 | Aluminiumhydrid (AlH3) |
| Kalium | +1 | Kaliumhydrid (KH) |
| Calcium | +2 | Calciumhydrid (CaH2) |
| Titan | +2 | Titandihydrid (TiH2) |
| Kupfer | +1 | Kupferhydrid (CuH) |
| Zink | +2 | Zink(II)-hydrid (ZnH2) |
| Rubidium | +1 | Rubidiumhydrid (RbH) |
| Strontium | +2 | Strontiumhydrid (SrH2) |
| Yttrium | +3 | Yttriumhydrid (YH3) |
| Zirconium | +2 | Zirconiumhydrid (ZrH2) |
| Cadmium | +2 | Cadmiumhydrid (CdH2) |
| Caesium | +1 | Caesiumhydrid (CsH) |
| Barium | +2 | Bariumhydrid (BaH2) |
| Europium | +2 | Europium(II)-hydrid (EuH2) |
| Quecksilber | +1 | Quecksilber(I)-hydrid (HgH) |
| +2 | Quecksilber(II)-hydrid (HgH2) | |
| Thallium | +3 | Thalliumhydrid (TlH3) |
| Uran | +3 | Uran(III)-hydrid (UH3) |
| Plutonium | +2 | Plutonium(II)-hydrid (PuH2) |
| Americium | +3 | Americium(III)-hydrid (AmH3) |
Halogenide
Viele bekannte Salze enthalten Halogene, also Fluor, Chlor, Brom oder Iod. Sie werden Fluoride, Chloride, Bromide, Iodide oder allgemein Halogenide genannt und sind von den Halogenwasserstoffen und ihren Säuren, den Halogenwasserstoffsäuren abgeleitet.
| Element (Kation) | Oxidationszustand | Fluorid | Chlorid | Bromid | Iodid |
|---|---|---|---|---|---|
| Lithium | +1 | Lithiumfluorid (LiF) | Lithiumchlorid (LiCl) | Lithiumbromid (LiBr) | Lithiumiodid (LiI) |
| Beryllium | +2 | Berylliumfluorid (BeF2) | Berylliumchlorid (BeCl2) | Berylliumbromid (BeBr2) | Berylliumiodid (BeI2) |
| Natrium | +1 | Natriumfluorid (NaF) | Natriumchlorid (NaCl) | Natriumbromid (NaBr) | Natriumiodid (NaI) |
| Magnesium | +2 | Magnesiumfluorid (MgF2) | Magnesiumchlorid (MgCl2) | Magnesiumbromid (MgBr2) | Magnesiumiodid (MgI2) |
| Aluminium | +1 | Aluminium(I)-fluorid (AlF) | Aluminium(I)-chlorid (AlCl) | Aluminium(I)-bromid (AlBr) | Aluminium(I)-iodid (AlI) |
| +3 | Aluminiumfluorid (AlF3) | Aluminiumchlorid (AlCl3) | Aluminiumbromid (AlBr3) | Aluminiumiodid (AlI3) | |
| Silicium | +4 | Siliciumtetrafluorid (SiF4) | Siliciumtetrachlorid (SiCl4) | Siliciumtetrabromid (SiBr4) | Siliciumtetraiodid (SiI4) |
| Kalium | +1 | Kaliumfluorid (KF) | Kaliumchlorid (KCl) | Kaliumbromid (KBr) | Kaliumiodid (KI) |
| Calcium | +2 | Calciumfluorid (CaF2) | Calciumchlorid (CaCl2) | Calciumbromid (CaBr2) | Calciumiodid (CaI2) |
| Scandium | +3 | Scandiumfluorid (ScF3) | Scandiumchlorid (ScCl3) | Scandiumbromid (ScBr3) | Scandiumiodid (ScI3) |
| Titan | +2 | Titan(II)-fluorid (TiF2) | Titan(II)-chlorid (TiCl2) | Titan(II)-bromid (TiBr2) | Titan(II)-iodid (TiI2) |
| +3 | Titan(III)-fluorid (TiF3) | Titan(III)-chlorid (TiCl3) | Titan(III)-bromid (TiBr3) | Titan(III)-iodid (TiI3) | |
| +4 | Titan(IV)-fluorid (TiF4) | Titan(IV)-chlorid (TiCl4) | Titan(IV)-bromid (TiBr4) | Titan(IV)-iodid (TiI4) | |
| Vanadium | +2 | Vanadium(II)-fluorid (VF2) | Vanadium(II)-chlorid (VCl2) | Vanadium(II)-bromid (VBr2) | Vanadium(II)-iodid (VI2) |
| +3 | Vanadium(III)-fluorid (VF3) | Vanadium(III)-chlorid (VCl3) | Vanadium(III)-bromid (VBr3) | Vanadium(III)-iodid (VI3) | |
| +4 | Vanadium(IV)-fluorid (VF4) | Vanadium(IV)-chlorid (VCl4) | Vanadium(IV)-bromid (VBr4) | Vanadium(IV)-iodid (VI4) | |
| Chrom | +2 | Chrom(II)-fluorid (CrF2) | Chrom(II)-chlorid (CrCl2) | Chrom(II)-bromid (CrBr2) | Chrom(II)-iodid (CrI2) |
| +3 | Chrom(III)-fluorid (CrF3) | Chrom(III)-chlorid (CrCl3) | Chrom(III)-bromid (CrBr3) | Chrom(III)-iodid (CrI3) | |
| +4 | Chrom(IV)-fluorid (CrF4) | Chrom(IV)-chlorid (CrCl4) | |||
| +5 | Chrom(V)-fluorid (CrF5) | ||||
| +6 | Chrom(VI)-fluorid (CrF6) | ||||
| Mangan | +2 | Mangan(II)-fluorid (MnF2) | Mangan(II)-chlorid (MnCl2) | Mangan(II)-bromid (MnBr2) | Mangan(II)-iodid (MnI2) |
| +3 | Mangan(III)-fluorid (MnF3) | Mangan(III)-chlorid (MnCl3) | Mangan(III)-bromid (MnBr3) | Mangan(III)-iodid (MnI3) | |
| +4 | Mangan(IV)-fluorid (MnF4) | ||||
| Eisen | +2 | Eisen(II)-fluorid (FeF2) | Eisen(II)-chlorid (FeCl2) | Eisen(II)-bromid (FeBr2) | Eisen(II)-iodid (FeI2) |
| +3 | Eisen(III)-fluorid (FeF3) | Eisen(III)-chlorid (FeCl3) | Eisen(III)-bromid (FeBr3) | Eisen(III)-iodid (FeI3) | |
| Cobalt | +2 | Cobalt(II)-fluorid (CoF2) | Cobalt(II)-chlorid (CoCl2) | Cobalt(II)-bromid (CoBr2) | Cobalt(II)-iodid (CoI2) |
| +3 | Cobalt(III)-fluorid (CoF3) | Cobalt(III)-chlorid (CoCl3) | Cobalt(III)-bromid (CoBr3) | Cobalt(III)-iodid (CoI3) | |
| Nickel | +2 | Nickel(II)-fluorid (NiF2) | Nickel(II)-chlorid (NiCl2) | Nickel(II)-bromid (NiBr2) | Nickel(II)-iodid (NiI2) |
| Kupfer | +1 | Kupfer(I)-fluorid (CuF) | Kupfer(I)-chlorid (CuCl) | Kupfer(I)-bromid (CuBr) | Kupfer(I)-iodid (CuI) |
| +2 | Kupfer(II)-fluorid (CuF2) | Kupfer(II)-chlorid (CuCl2) | Kupfer(II)-bromid (CuBr2) | Kupfer(II)-iodid (CuI2) | |
| Zink | +2 | Zinkfluorid (ZnF2) | Zinkchlorid (ZnCl2) | Zinkbromid (ZnBr2) | Zinkiodid (ZnI2) |
| Gallium | +3 | Gallium(III)-fluorid (GaF3) | Gallium(III)-chlorid (GaCl3) | Gallium(III)-bromid (GaBr3) | Gallium(III)-iodid (GaI3) |
| Germanium | +2 | Germanium(II)-fluorid (GeF2) | Germanium(II)-chlorid (GeCl2) | Germanium(II)-bromid (GeBr2) | Germanium(II)-iodid (GeI2) |
| +4 | Germanium(IV)-fluorid (GeF4) | Germanium(IV)-chlorid (GeCl4) | Germanium(IV)-bromid (GeBr4) | Germanium(IV)-iodid (GeI4) | |
| Arsen | +3 | Arsen(III)-fluorid (AsF3) | Arsen(III)-chlorid (AsCl3) | Arsen(III)-bromid (AsBr3) | Arsen(III)-iodid (AsI3) |
| +5 | Arsen(V)-fluorid (AsF5) | Arsen(V)-chlorid (AsCl5) | Arsen(V)-bromid (AsBr5) | Arsen(V)-iodid (AsI5) | |
| Selen | +4 | Selentetrafluorid (SeF4) | Selentetrachlorid (SeCl4) | Selentetrabromid (SeBr4) | Selentetraiodid (SeI4) |
| Krypton | +2 | Kryptondifluorid (KrF2) | |||
| Rubidium | +1 | Rubidiumfluorid (RbF) | Rubidiumchlorid (RbCl) | Rubidiumbromid (RbBr) | Rubidiumiodid (RbI) |
| Strontium | +2 | Strontiumfluorid (SrF2) | Strontiumchlorid (SrCl2) | Strontiumbromid (SrBr2) | Strontiumiodid (SrI2) |
| Yttrium | +3 | Yttrium(III)-fluorid (YF3) | Yttrium(III)-chlorid (YCl3) | Yttrium(III)-bromid (YBr3) | Yttrium(III)-iodid (YI3) |
| Zirconium | +3 | Zirconium(III)-fluorid (ZrF3) | Zirconium(III)-chlorid (ZrCl3) | Zirconium(III)-bromid (ZrBr3) | Zirconium(III)-iodid (ZrI3) |
| +4 | Zirconium(IV)-fluorid (ZrF4) | Zirconium(IV)-chlorid (ZrCl4) | Zirconium(IV)-bromid ZrBr4) | Zirconium(IV)-iodid (ZrI4) | |
| Niob | +4 | Niob(IV)-fluorid (NbF4) | Niob(IV)-chlorid (NbCl4) | Niob(IV)-bromid (NbBr4) | Niob(IV)-iodid (NbI4) |
| +5 | Niob(V)-fluorid (NbF5) | Niob(V)-chlorid (NbCl5) | Niob(V)-bromid (NbBr5) | Niob(V)-iodid (NbI5) | |
| Molybdän | +2 | Molybdän(II)-fluorid (MoF2) | Molybdän(II)-chlorid (MoCl2) | Molybdän(II)-bromid (MoBr2) | Molybdän(II)-iodid (MoI2) |
| +3 | Molybdän(III)-fluorid (MoF3) | Molybdän(III)-chlorid (MoCl3) | Molybdän(III)-bromid (MoBr3) | Molybdän(III)-iodid (MoI3) | |
| +4 | Molybdän(IV)-fluorid (MoF4) | Molybdän(IV)-chlorid (MoCl4) | Molybdän(IV)-bromid (MoBr4) | Molybdän(IV)-iodid (MoI4) | |
| +5 | Molybdän(V)-fluorid (MoF5) | Molybdän(V)-chlorid (MoCl5) | |||
| +6 | Molybdän(VI)-fluorid (MoF6) | Molybdän(VI)-chlorid (MoCl6) | |||
| Ruthenium | +3 | Ruthenium(III)-fluorid (RuF3) | Ruthenium(III)-chlorid (RuCl3) | Ruthenium(III)-bromid (RuBr3) | Ruthenium(III)-iodid (RuI3) |
| +5 | Ruthenium(V)-fluorid (RuF5) | ||||
| +6 | Ruthenium(VI)-fluorid (RuF6) | ||||
| Rhodium | +3 | Rhodium(III)-fluorid (RhF3) | Rhodium(III)-chlorid (RhCl3) | Rhodium(III)-bromid (RhBr3) | Rhodium(III)-iodid (RhI3) |
| +6 | Rhodium(VI)-fluorid (RhF6) | ||||
| Palladium | +2 | Palladium(II)-fluorid (PdF2) | Palladium(II)-chlorid (PdCl2) | Palladium(II)-bromid (PdBr2) | Palladium(II)-iodid (PdI2) |
| +2 / +4 | Palladium(II,IV)-fluorid (PdF3) | ||||
| Silber | +1 | Silber(I)-fluorid (AgF) | Silberchlorid (AgCl) | Silberbromid (AgBr) | Silberiodid (AgI) |
| +2 | Silber(II)-fluorid (AgF2) | ||||
| Cadmium | +2 | Cadmiumfluorid (CdF2) | Cadmiumchlorid (CdCl2) | Cadmiumbromid (CdBr2) | Cadmiumiodid (CdI2) |
| Indium | +1 | Indium(I)-fluorid (InF) | Indium(I)-chlorid (InCl) | Indium(I)-bromid (InBr) | Indium(I)-iodid (InI) |
| +3 | Indium(III)-fluorid (InF3) | Indium(III)-chlorid (InCl3) | Indium(III)-bromid (InBr3) | Indium(III)-iodid (InI3) | |
| Zinn | +2 | Zinn(II)-fluorid (SnF2) | Zinn(II)-chlorid (SnCl2) | Zinn(II)-bromid (SnBr2) | Zinn(II)-iodid (SnI2) |
| +4 | Zinn(IV)-fluorid (SnF4) | Zinn(IV)-chlorid (SnCl4) | Zinn(IV)-bromid (SnBr4) | Zinn(IV)-iodid (SnI4) | |
| Antimon | +3 | Antimon(III)-fluorid (SbF3) | Antimon(III)-chlorid (SbCl3) | Antimon(III)-bromid (SbBr3) | Antimon(III)-iodid (SbI3) |
| +5 | Antimon(V)-fluorid (SbF5) | Antimon(V)-chlorid (SbCl5) | |||
| Tellur | +1 | Tellur(I)-fluorid (TeF) | Tellur(I)-chlorid (TeCl) | Tellur(I)-bromid (TeBr) | Tellur(I)-iodid (TeI) |
| +4 | Tellurtetrafluorid (TeF4) | Tellurtetrachlorid (TeCl4) | Tellurtetrabromid (TeBr4) | Tellurtetraiodid (TeI4) | |
| +6 | Tellurhexafluorid (TeF6) | ||||
| Xenon | +2 | Xenondifluorid (XeF2) | Xenondichlorid (XeCl2) | ||
| +4 | Xenontetrafluorid (XeF4) | ||||
| +6 | Xenonhexafluorid (XeF6) | ||||
| Caesium | +1 | Caesiumfluorid (CsF) | Caesiumchlorid (CsCl) | Caesiumbromid (CsBr) | Caesiumiodid (CsI) |
| Barium | +2 | Bariumfluorid (BaF2) | Bariumchlorid (BaCl2) | Bariumbromid (BaBr2) | Bariumiodid (BaI2) |
| Lanthan | +3 | Lanthanfluorid (LaF3) | Lanthanchlorid (LaCl3) | Lanthanbromid (LaBr3) | Lanthaniodid (LaI3) |
| Cer | +3 | Cer(III)-fluorid (CeF3) | Cer(III)-chlorid (CeCl3) | Cer(III)-bromid (CeBr3) | Cer(III)-iodid (CeI3) |
| Praseodym | +3 | Praseodym(III)-fluorid (PrF3) | Praseodym(III)-chlorid (PrCl3) | Praseodym(III)-bromid (PrBr3) | Praseodym(III)-iodid (PrI3) |
| Neodym | +2 | Neodym(II)-fluorid (NdF2) | Neodym(II)-chlorid (NdCl2) | Neodym(II)-bromid (NdBr2) | Neodym(II)-iodid (NdI2) |
| +3 | Neodym(III)-fluorid (NdF3) | Neodym(III)-chlorid (NdCl3) | Neodym(III)-bromid (NdBr3) | Neodym(III)-iodid (NdI3) | |
| Promethium | +3 | Promethium(III)-fluorid (PmF3) | Promethium(III)-chlorid (PmCl3) | Promethium(III)-bromid (PmBr3) | Promethium(III)-iodid (PmI3) |
| Samarium | +2 | Samarium(II)-fluorid (SmF2) | Samarium(II)-chlorid (SmCl2) | Samarium(II)-bromid (SmBr2) | Samarium(II)-iodid (SmI2) |
| +3 | Samarium(III)-fluorid (SmF3) | Samarium(III)-chlorid (SmCl3) | Samarium(III)-bromid (SmBr3) | Samarium(III)-iodid (SmI3) | |
| Europium | +2 | Europium(II)-fluorid (EuF2) | Europium(II)-chlorid (EuCl2) | Europium(II)-bromid (EuBr2) | Europium(II)-iodid (EuI2) |
| +3 | Europium(III)-fluorid (EuF3) | Europium(III)-chlorid (EuCl3) | Europium(III)-bromid (EuBr3) | Europium(III)-iodid (EuI3) | |
| Gadolinium | +3 | Gadolinium(III)-fluorid (GdF3) | Gadolinium(III)-chlorid (GdCl3) | Gadolinium(III)-bromid (GdBr3) | Gadolinium(III)-iodid (GdI3) |
| Terbium | +3 | Terbium(III)-fluorid (TbF3) | Terbium(III)-chlorid (TbCl3) | Terbium(III)-bromid (TbBr3) | Terbium(III)-iodid (TbI3) |
| Dysprosium | +2 | Dysprosium(II)-fluorid (DyF2) | Dysprosium(II)-chlorid (DyCl2) | Dysprosium(II)-bromid (DyBr2) | Dysprosium(II)-iodid (DyI2) |
| +3 | Dysprosium(III)-fluorid (DyF3) | Dysprosium(III)-chlorid (DyCl3) | Dysprosium(III)-bromid (DyBr3) | Dysprosium(III)-iodid (DyI3) | |
| Holmium | +3 | Holmium(III)-fluorid (HoF3) | Holmium(III)-chlorid (HoCl3) | Holmium(III)-bromid (HoBr3) | Holmium(III)-iodid (HoI3) |
| Erbium | +3 | Erbium(III)-fluorid (ErF3) | Erbium(III)-chlorid (ErCl3) | Erbium(III)-bromid (ErBr3) | Erbium(III)-iodid (ErI3) |
| Thulium | +2 | Thulium(II)-fluorid (TmF2) | Thulium(II)-chlorid (TmCl2) | Thulium(II)-bromid (TmBr2) | Thulium(II)-iodid (TmI2) |
| +3 | Thulium(III)-fluorid (TmF3) | Thulium(III)-chlorid (TmCl3) | Thulium(III)-bromid (TmBr3) | Thulium(III)-iodid (TmI3) | |
| Ytterbium | +2 | Ytterbium(II)-fluorid (YbF2) | Ytterbium(II)-chlorid (YbCl2) | Ytterbium(II)-bromid (YbBr2) | Ytterbium(II)-iodid (YbI2) |
| +3 | Ytterbium(III)-fluorid (YbF3) | Ytterbium(III)-chlorid (YbCl3) | Ytterbium(III)-bromid (YbBr3) | Ytterbium(III)-iodid (YbI3) | |
| Lutetium | +3 | Lutetium(III)-fluorid (LuF3) | Lutetium(III)-chlorid (LuCl3) | Lutetium(III)-bromid (LuBr3) | Lutetium(III)-iodid (LuI3) |
| Hafnium | +4 | Hafnium(IV)-fluorid (HfF4) | Hafnium(IV)-chlorid (HfCl4) | Hafnium(IV)-bromid (HfBr4) | Hafnium(IV)-iodid (HfI4) |
| Tantal | +5 | Tantal(V)-fluorid (TaF5) | Tantal(V)-chlorid (TaCl5) | Tantal(V)-bromid (TaBr5) | Tantal(V)-iodid (TaI5) |
| Wolfram | +2 | Wolfram(II)-fluorid (WF2) | Wolfram(II)-chlorid (WCl2) | Wolfram(II)-bromid (WBr2) | Wolfram(II)-iodid (WI2) |
| +3 | Wolfram(III)-fluorid (WF3) | Wolfram(III)-chlorid (WCl3) | Wolfram(III)-bromid (WBr3) | Wolfram(III)-iodid (WI3) | |
| +4 | Wolfram(IV)-fluorid (WF4) | Wolfram(IV)-chlorid (WCl4) | Wolfram(IV)-bromid (WBr4) | Wolfram(IV)-iodid (WI4) | |
| +5 | Wolfram(V)-fluorid (WF5) | Wolfram(V)-chlorid (WCl5) | Wolfram(V)-bromid (WBr5) | Wolfram(V)-iodid (WI5) | |
| +6 | Wolfram(VI)-fluorid (WF6) | Wolfram(VI)-chlorid (WCl6) | Wolfram(VI)-bromid (WBr6) | Wolfram(VI)-iodid (WI6) | |
| Rhenium | +3 | Rhenium(III)-fluorid (ReF3) | Rhenium(III)-chlorid (ReCl3) | Rhenium(III)-bromid (ReBr3) | Rhenium(III)-iodid (ReI3) |
| +4 | Rhenium(IV)-fluorid (ReF4) | Rhenium(IV)-chlorid (ReCl4) | Rhenium(IV)-bromid (ReBr4) | Rhenium(IV)-iodid (ReI4) | |
| +5 | Rhenium(V)-fluorid (ReF5) | Rhenium(V)-chlorid (ReCl5) | Rhenium(V)-bromid (ReBr5) | ||
| +6 | Rhenium(VI)-fluorid (ReF6) | ||||
| +7 | Rhenium(VII)-fluorid (ReF7) | ||||
| Platin | +2 | Platin(II)-fluorid (PtF2) | Platin(II)-chlorid (PtCl2) | Platin(II)-bromid (PtBr2) | Platin(II)-iodid (PtI2) |
| +4 | Platin(IV)-fluorid (PtF4) | Platin(IV)-chlorid (PtCl4) | Platin(IV)-bromid (PtBr4) | Platin(IV)-iodid (PtI4) | |
| +5 | Platin(V)-fluorid (PtF5) | ||||
| +6 | Platin(VI)-fluorid (PtF6) | ||||
| Gold | +1 | Gold(I)-fluorid (AuF) | Gold(I)-chlorid (AuCl) | Gold(I)-bromid (AuBr) | Gold(I)-iodid (AuI) |
| +3 | Gold(III)-fluorid (AuF3) | Gold(III)-chlorid (AuCl3) | Gold(III)-bromid (AuBr3) | Gold(III)-iodid (AuI3) | |
| +5 | Gold(V)-fluorid (AuF5) | ||||
| Quecksilber | +1 | Quecksilber(I)-fluorid (HgF) | Quecksilber(I)-chlorid (HgCl) | Quecksilber(I)-bromid (HgBr) | Quecksilber(I)-iodid (HgI) |
| +2 | Quecksilber(II)-fluorid (HgF2) | Quecksilber(II)-chlorid (HgCl2) | Quecksilber(II)-bromid (HgBr2) | Quecksilber(II)-iodid (HgI2) | |
| Thallium | +1 | Thallium(I)-fluorid (TlF) | Thallium(I)-chlorid (TlCl) | Thallium(I)-bromid (TlBr) | Thallium(I)-iodid (TlI) Thallium(I)-triiodid (TlI3) |
| +3 | Thallium(III)-fluorid (TlF3) | Thallium(III)-chlorid (TlCl3) | Thallium(III)-bromid (TlBr3) | ||
| Blei | +2 | Blei(II)-fluorid (PbF2) | Blei(II)-chlorid (PbCl2) | Blei(II)-bromid (PbBr2) | Blei(II)-iodid (PbI2) |
| +4 | Blei(IV)-fluorid (PbF4) | Blei(IV)-chlorid (PbCl4) | |||
| Bismut | +3 | Bismut(III)-fluorid (BiF3) | Bismut(III)-chlorid (BiCl3) | Bismut(III)-bromid (BiBr3) | Bismut(III)-iodid (BiI3) |
| +5 | Bismut(V)-fluorid (BiF5) | ||||
| Radon | +2 | Radon(II)-fluorid (RnF2) | |||
| Radium | +2 | Radiumfluorid (RaF2) | Radiumchlorid (RaCl2) | Radiumbromid (RaBr2) | Radiumiodid (RaI2) |
| Actinium | +3 | Actinium(III)-fluorid (AcF3) | Actinium(III)-chlorid (AcCl3) | Actinium(III)-bromid (AcBr3) | Actinium(III)-iodid (AcI3) |
| Thorium | +4 | Thorium(IV)-fluorid (ThF4) | Thorium(IV)-chlorid (ThCl4) | Thorium(IV)-bromid (ThBr4) | Thorium(IV)-iodid (ThI4) |
| Protactinium | +5 | Protactinium(V)-fluorid (PaF5) | Protactinium(V)-chlorid (PaCl5) | Protactinium(V)-bromid (PaBr5) | Protactinium(V)-iodid (PaI5) |
| Uran | +3 | Uran(III)-fluorid (UF3) | Uran(III)-chlorid (UCl3) | Uran(III)-bromid (UBr3) | Uran(III)-iodid (UI3) |
| +4 | Uran(IV)-fluorid (UF4) | Uran(IV)-chlorid (UCl4) | Uran(IV)-bromid (UBr4) | Uran(IV)-iodid (UI4) | |
| +5 | Uran(V)-fluorid (UF5) | Uran(V)-chlorid (UCl5) | Uran(V)-bromid (UBr5) | Uran(V)-iodid (UI5) | |
| +6 | Uran(VI)-fluorid (UF6) | Uran(VI)-chlorid (UCl6) | |||
| Neptunium | +3 | Neptunium(III)-fluorid (NpF3) | Neptunium(III)-chlorid (NpCl3) | Neptunium(III)-bromid (NpBr3) | Neptunium(III)-iodid (NpI3) |
| +4 | Neptunium(IV)-fluorid (NpF4) | Neptunium(IV)-chlorid (NpCl4) | Neptunium(IV)-bromid (NpBr4) | ||
| +5 | Neptunium(V)-fluorid (NpF5) | ||||
| +6 | Neptunium(VI)-fluorid (NpF6) | ||||
| Plutonium | +3 | Plutonium(III)-fluorid (PuF3) | Plutonium(III)-chlorid (PuCl3) | Plutonium(III)-bromid (PuBr3) | Plutonium(III)-iodid (PuI3) |
| +4 | Plutonium(IV)-fluorid (PuF4) | ||||
| +5 | Plutonium(V)-fluorid (PuF5) | ||||
| +6 | Plutonium(VI)-fluorid (PuF6) |
Chalkogenide
Die Salze, die Elemente der 6. Hauptgruppe des Periodensystems, die Chalkogene, enthalten, werden Oxide, Sulfide, Selenide, Telluride oder allgemein Chalkogenide genannt.
| Element (Kation) | Oxidationszustand | Oxid | Sulfid | Selenid | Tellurid |
|---|---|---|---|---|---|
| Lithium | +1 | Lithiumoxid (Li2O) | Lithiumsulfid (Li2S) | Lithiumselenid (Li2Se) | Lithiumtellurid (Li2Te) |
| Beryllium | +2 | Berylliumoxid (BeO) | Berylliumsulfid (BeS) | Berylliumselenid (BeSe) | Berylliumtellurid (BeTe) |
| Bor | +3 | Bortrioxid (B2O3) | Bortrisulfid (B2S3) | ||
| Natrium | +1 | Natriumoxid (Na2O) | Natriumsulfid (Na2S) | Natriumselenid (Na2Se) | Natriumtellurid (Na2Te) |
| Natriumperoxid (Na2O2) | |||||
| Natriumhyperoxid (NaO2) | |||||
| Magnesium | +2 | Magnesiumoxid (MgO) | Magnesiumsulfid (MgS) | Magnesiumselenid (MgSe) | Magnesiumtellurid (MgTe) |
| Magnesiumperoxid (MgO2) | |||||
| Aluminium | +3 | Aluminiumoxid (Al2O3) | Aluminiumsulfid (Al2S3) | Aluminiumselenid (Al2Se3) | Aluminiumtellurid (Al2Te3) |
| Silicium | +4 | Siliciumdioxid (SiO2) | Siliciumdisulfid (SiS2) | ||
| Kalium | +1 | Kaliumoxid (K2O) | Kaliumsulfid (K2S) | Kaliumselenid (K2Se) | Kaliumtellurid (K2Te) |
| Kaliumperoxid (K2O2) | |||||
| Kaliumhyperoxid (KO2) | |||||
| Kaliumozonid (KO3) | |||||
| Calcium | +2 | Calciumoxid (CaO) | Calciumsulfid (CaS) | Calciumselenid (CaSe) | Calciumtellurid (CaTe) |
| Calciumperoxid (CaO2) | |||||
| Scandium | +3 | Scandiumoxid (Sc2O3) | Scandiumsulfid (Sc2S3) | ||
| Titan | +2 | Titan(II)-oxid (TiO) | Titan(II)-sulfid (TiS) | ||
| +3 | Titan(III)-oxid (Ti2O3) | Titan(III)-sulfid (Ti2S3) | |||
| +4 | Titan(IV)-oxid (TiO2) | Titan(IV)-sulfid (TiS2) | |||
| Vanadium | +2 | Vanadium(II)-oxid (VO) | Vanadium(II)-sulfid (VS) | ||
| +3 | Vanadium(III)-oxid (V2O3) | Vanadium(III)-sulfid (V2S3) | |||
| +4 | Vanadium(IV)-oxid (VO2) | Vanadium(IV)-sulfid (VS2) | |||
| +5 | Vanadium(V)-oxid (V2O5) | Vanadium(V)-sulfid (V2S5) | |||
| Chrom | +2 | Chrom(II)-oxid (CrO) | Chrom(II)-sulfid (CrS) | ||
| +3 | Chrom(III)-oxid (Cr2O3) | Chrom(III)-sulfid (Cr2S3) | |||
| +4 | Chrom(IV)-oxid (CrO2) | ||||
| +6 | Chrom(VI)-oxid (CrO3) | ||||
| Mangan | +2 | Mangan(II)-oxid (MnO) | Mangan(II)-sulfid (MnS) | ||
| +3 | Mangan(III)-oxid (Mn2O3) | ||||
| +4 | Mangan(IV)-oxid (MnO2) | ||||
| Eisen | +2 | Eisen(II)-oxid (FeO) | Eisen(II)-sulfid (FeS) Eisen(II)-disulfid (FeS2) | ||
| +2 / +3 | Eisen(II,III)-oxid (Fe3O4) | Eisen(II,III)-sulfid (Fe3S4) | |||
| +3 | Eisen(III)-oxid (Fe2O3) | Eisen(III)-sulfid (Fe2S3) | |||
| Cobalt | +2 | Cobalt(II)-oxid (CoO) | Cobalt(II)-sulfid (CoS) | ||
| +2 / +3 | Cobalt(II,III)-oxid (Co3O4) | Cobalt(II,III)-sulfid (Co3S4) | |||
| +3 | Cobalt(III)-oxid (Co2O3) | Cobalt(III)-sulfid (Co2S3) | |||
| Nickel | +2 | Nickel(II)-oxid (NiO) | Nickel(II)-sulfid (NiS) | ||
| +3 | Nickel(III)-oxid (Ni2O3) | Nickel(III)-sulfid (Ni2S3) | |||
| +4 | Nickel(IV)-oxid (NiO2) | Nickeldisulfid (NiS2) | |||
| Kupfer | +1 | Kupfer(I)-oxid (Cu2O) | Kupfer(I)-sulfid (Cu2S) | Kupfer(I)-selenid (Cu2Se) | Kupfer(I)-tellurid (Cu2Te) |
| +2 | Kupfer(II)-oxid (CuO) | Kupfer(II)-sulfid (CuS) | Kupfer(II)-selenid (CuSe) | Kupfer(II)-tellurid (CuTe) | |
| Zink | +2 | Zinkoxid (ZnO) | Zinksulfid (ZnS) | Zinkselenid (ZnSe) | Zinktellurid (ZnTe) |
| Gallium | +1 | Gallium(I)-oxid (Ga2O) | Gallium(I)-sulfid (Ga2S) | Gallium(I)-selenid (Ga2Se) | Gallium(I)-tellurid (Ga2Te) |
| +3 | Gallium(III)-oxid (Ga2O3) | Gallium(III)-sulfid (Ga2S3) | Gallium(III)-selenid (Ga2Se3) | Gallium(III)-tellurid (Ga2Te3) | |
| Germanium | +2 | Germanium(II)-oxid (GeO) | Germanium(II)-sulfid (GeS) | Germanium(II)-selenid (GeSe) | Germanium(II)-tellurid (GeTe) |
| +4 | Germanium(IV)-oxid (GeO2) | Germanium(IV)-sulfid (GeS2) | |||
| Arsen | +3 | Arsen(III)-oxid (As2O3) | Arsen(III)-sulfid (As2S3) | Arsen(III)-selenid (As2Se3) | Arsen(III)-tellurid (As2Te3) |
| +5 | Arsen(V)-oxid (As2O5) | Arsen(V)-sulfid (As2S5) | |||
| Selen | +4 | Selen(IV)-oxid (SeO2) | Selen(IV)-sulfid (SeS2) | ||
| +6 | Selen(VI)-oxid (SeO3) | ||||
| Rubidium | +1 | Rubidiumoxid (Rb2O) | Rubidiumsulfid (Rb2S) | Rubidiumselenid (Rb2Se) | Rubidiumtellurid (Rb2Te) |
| Rubidiumperoxid (Rb2O2) | |||||
| Rubidiumhyperoxid (RbO2) | |||||
| Rubidiumozonid (RbO3) | |||||
| Strontium | +2 | Strontiumoxid (SrO) | Strontiumsulfid (SrS) | Strontiumselenid (SrSe) | Strontiumtellurid (SrTe) |
| Strontiumperoxid (SrO2) | |||||
| Yttrium | +3 | Yttrium(III)-oxid (Y2O3) | Yttrium(III)-sulfid (Y2S3) | ||
| Zirconium | +4 | Zirconium(IV)-oxid (ZrO2) | |||
| Niob | +2 | Niob(II)-oxid (NbO) | |||
| +4 | Niob(IV)-oxid (NbO2) | Niob(IV)-sulfid (NbS2) | |||
| +5 | Niob(V)-oxid (Nb2O5) | ||||
| Molybdän | +2 | Molybdän(II)-oxid (MoO) | |||
| +4 | Molybdän(IV)-oxid (MoO2) | Molybdän(IV)-sulfid (MoS2) | |||
| +6 | Molybdän(VI)-oxid (MoO3) | ||||
| Ruthenium | +4 | Ruthenium(IV)-oxid (RuO2) | |||
| +8 | Ruthenium(VIII)-oxid (RuO4) | ||||
| Rhodium | +3 | Rhodium(III)-oxid (Rh2O3) | |||
| +4 | Rhodium(IV)-oxid (RhO2) | ||||
| Palladium | +2 | Palladium(II)-oxid (PdO) | |||
| +4 | Palladium(IV)-oxid (PdO2) | ||||
| Silber | +1 | Silber(I)-oxid (Ag2O) | Silbersulfid (Ag2S) | Silber(I)-selenid (Ag2Se) | Silbertellurid (Ag2Te) |
| +1 / +3 | Silber(I,III)-oxid (AgO) | ||||
| Cadmium | +2 | Cadmiumoxid (CdO) | Cadmiumsulfid (CdS) | Cadmiumselenid (CdSe) | Cadmiumtellurid (CdTe) |
| Indium | +3 | Indium(III)-oxid (In2O3) | Indium(III)-sulfid (In2S3) | Indium(III)-selenid (In2Se3) | Indium(III)-tellurid (In2Te3) |
| Zinn | +2 | Zinn(II)-oxid (SnO) | Zinn(II)-sulfid (SnS) | Zinn(II)-selenid (SnSe) | Zinn(II)-tellurid (SnTe) |
| +4 | Zinn(IV)-oxid (SnO2) | Zinn(IV)-sulfid (SnS2) | |||
| Antimon | +3 | Antimon(III)-oxid (Sb2O3) | Antimon(III)-sulfid (Sb2S3) | Antimon(III)-selenid (Sb2Se3) | Antimon(III)-tellurid (Sb2Te3) |
| +3 / +5 | Antimon(III,V)-oxid (Sb2O4) | ||||
| +5 | Antimon(V)-oxid (Sb2O5) | Antimon(V)-sulfid (Sb2S5) | |||
| Tellur | +4 | Tellurdioxid (TeO2) | |||
| +6 | Tellurtrioxid (TeO3) | ||||
| Xenon | +6 | Xenon(VI)-oxid (XeO3) | |||
| +8 | Xenon(VIII)-oxid (XeO4) | ||||
| Caesium | +1 | Caesiumoxid (Cs2O) | |||
| Caesiumperoxid (Cs2O2) | |||||
| Caesiumhyperoxid (CsO2) | |||||
| Caesiumozonid (CsO3) | |||||
| Barium | +2 | Bariumoxid (BaO) | Bariumsulfid (BaS) | Bariumselenid (BaSe) | Bariumtellurid (BaTe) |
| Bariumperoxid (BaO2) | |||||
| Lanthan | +3 | Lanthanoxid (La2O3) | |||
| Cer | +3 | Cer(III)-oxid (Ce2O3) | |||
| +4 | Cer(IV)-oxid (CeO2) | ||||
| Praseodym | +3 | Praseodym(III)-oxid (Pr2O3) | Praseodym(III)-sulfid (Pr2S3) | ||
| +3 / +4 | Praseodym(III,IV)-oxid (Pr6O11) | ||||
| +4 | Praseodym(IV)-oxid (PrO2) | ||||
| Neodym | +3 | Neodym(III)-oxid (Nd2O3) | Neodym(III)-sulfid (Nd2S3) | ||
| Promethium | +3 | Promethium(III)-oxid (Pm2O3) | |||
| Samarium | +3 | Samarium(III)-oxid (Sm2O3) | Samarium(III)-sulfid (Sm2S3) | ||
| Europium | +2 | Europium(II)-oxid (EuO) | Europium(II)-sulfid (EuS) | Europium(II)-selenid (EuSe) | Europium(II)-tellurid (EuTe) |
| +3 | Europium(III)-oxid (Eu2O3) | ||||
| Gadolinium | +3 | Gadolinium(III)-oxid (Gd2O3) | |||
| Terbium | +3 | Terbium(III)-oxid (Tb2O3) | |||
| +3 / +4 | Terbium(III,IV)-oxid (Tb4O7) | ||||
| +4 | Terbium(IV)-oxid (TbO2) | ||||
| Dysprosium | +3 | Dysprosium(III)-oxid (Dy2O3) | |||
| Holmium | +3 | Holmium(III)-oxid (Ho2O3) | Holmium(III)-sulfid (Ho2S3) | ||
| Erbium | +3 | Erbium(III)-oxid (Er2O3) | |||
| Thulium | +3 | Thulium(III)-oxid (Tm2O3) | |||
| Ytterbium | +3 | Ytterbium(III)-oxid (Yb2O3) | |||
| Lutetium | +3 | Lutetium(III)-oxid (Lu2O3) | Lutetium(III)-sulfid (Lu2S3) | ||
| Hafnium | +4 | Hafnium(IV)-oxid (HfO2) | Hafnium(IV)-sulfid (HfS2) | ||
| Tantal | +4 | Tantal(IV)-sulfid (TaS2) | Tantal(IV)-selenid (TaSe2) | ||
| +5 | Tantal(V)-oxid (Ta2O5) | ||||
| Wolfram | +4 | Wolfram(IV)-oxid (WO2) | Wolfram(IV)-sulfid (WS2) | Wolfram(IV)-selenid (WSe2) | Wolframtellurid (WTe2) |
| +6 | Wolfram(VI)-oxid (WO3) | ||||
| Rhenium | +4 | Rhenium(IV)-oxid (ReO2) | Rhenium(IV)-sulfid (ReS2) | ||
| +6 | Rhenium(VI)-oxid (ReO3) | ||||
| +7 | Rhenium(VII)-oxid (Re2O7) | Rhenium(VII)-sulfid (Re2S7) | |||
| Osmium | +4 | Osmium(IV)-oxid (OsO2) | |||
| +8 | Osmium(VIII)-oxid (OsO4) | ||||
| Iridium | +4 | Iridium(IV)-oxid (IrO2) | |||
| Platin | +2 | Platin(II)-oxid (PtO) | Platin(IV)-oxid (PtS) | ||
| +4 | Platin(II)-sulfid (PtO2) | Platin(IV)-sulfid (PtS2) | |||
| Gold | +1 | Gold(I)-sulfid (Au2S) | |||
| +3 | Gold(III)-oxid (Au2O3) | Gold(III)-sulfid (Au2S3) | |||
| Quecksilber | +1 | Quecksilber(I)-oxid (Hg2O) | |||
| +2 | Quecksilber(II)-oxid (HgO) | Quecksilbersulfid (HgS) | Quecksilberselenid (HgSe) | Quecksilbertellurid (HgTe) | |
| Thallium | +1 | Thallium(I)-oxid (Tl2O) | Thallium(I)-sulfid (Tl2S) | ||
| +3 | Thallium(III)-oxid (Tl2O3) | ||||
| Blei | +2 | Blei(II)-oxid (PbO) | Blei(II)-sulfid (PbS) | Bleiselenid (PbSe) | Bleitellurid (PbTe) |
| +2 / +4 | Blei(II,IV)-oxid (Pb3O4) | ||||
| +4 | Blei(IV)-oxid (PbO2) | ||||
| Bismut | +3 | Bismut(III)-oxid (Bi2O3) | Bismut(III)-sulfid (Bi2S3) | Bismutselenid (Bi2Se3) | Bismuttellurid (Bi2Te3) |
| Actinium | +3 | Actinium(III)-oxid (Ac2O3) | Actinium(III)-sulfid (Ac2S3) | ||
| Thorium | +4 | Thorium(IV)-oxid (ThO2) | Thorium(IV)-sulfid (ThS2) | ||
| Protactinium | +4 | Protactinium(IV)-oxid (PaO2) | |||
| +5 | Protactinium(V)-oxid (Pa2O5) | ||||
| Uran | +4 | Uran(IV)-oxid (UO2) | |||
| +5 / +6 | Uran(V,VI)-oxid (U3O8) | ||||
| +6 | Uran(VI)-oxid (UO3) | ||||
| Neptunium | +4 | Neptunium(IV)-oxid (NpO2) | |||
| Plutonium | +4 | Plutonium(IV)-oxid (PuO2) |
Nitride, Phosphide, Arsenide und Antimonide
Die Salze, die Elemente der 5. Hauptgruppe des Periodensystems enthalten, sind die Nitride, Phosphide, Arsenide und Antimonide.
| Element (Kation) | Oxidationszustand | Nitrid | Phosphid | Arsenid | Antimonid |
|---|---|---|---|---|---|
| Lithium | +1 | Lithiumnitrid (Li3N) | |||
| Beryllium | +2 | Berylliumnitrid (Be3N2) | |||
| Bor | +3 | Bornitrid (BN) | Borphosphid (BP) | Borarsenid (BAs) Borsubarsenid (B12As2) | |
| Natrium | +1 | Natriumnitrid (Na3N) | Natriumphosphid (Na3P) | Natriumarsenid (Na3As) | Natriumantimonid (Na3Sb) |
| Magnesium | +2 | Magnesiumnitrid (Mg3N2) | Magnesiumphosphid (Mg3P2) | ||
| Aluminium | +3 | Aluminiumnitrid (AlN) | Aluminiumphosphid (AlP) | Aluminiumarsenid (AlAs) | Aluminiumantimonid (AlSb) |
| Silicium | +4 | Siliciumnitrid (Si3N4) | |||
| Kalium | +1 | Kaliumnitrid (K3N) | Kaliumphosphid (K3P) | Kaliumarsenid (K3As) | Kaliumantimonid (K3Sb) |
| Calcium | +2 | Calciumnitrid (Ca3N2) | Calciumphosphid (Ca3P2) | ||
| Scandium | +3 | Scandiumnitrid (ScN) | Scandiumphosphid (ScP) | ||
| Titan | +3 | Titannitrid (TiN) | Titanphosphid (TiP) | ||
| Vanadium | +3 | Vanadiumnitrid (VN) | |||
| Chrom | +3 | Chromnitrid (CrN) | |||
| Kupfer | +1 | Kupfer(I)-nitrid (Cu3N) | |||
| Zink | +2 | Zinknitrid (Zn3N2) | Zinkphosphid (Zn3P2) | Zinkarsenid (Zn3As2) | Zinkantimonid (Zn3Sb2) |
| Gallium | +3 | Galliumnitrid (GaN) | Galliumphosphid (GaP) | Galliumarsenid (GaAs) | Galliumantimonid (GaSb) |
| Germanium | +4 | Germaniumnitrid(Ge3N4) | |||
| Strontium | +2 | Strontiumnitrid (Sr3N2) | |||
| Yttrium | +3 | Yttriumnitrid (YN) | |||
| Zirconium | +3 | Zirconiumnitrid (ZrN) | |||
| Niob | +3 | Niobnitrid (NbN) | |||
| Silber | +1 | Silbernitrid (Ag3N) | |||
| Cadmium | +2 | Cadmiumnitrid (Cd3N2) | Cadmiumarsenid (Cd3As2) | ||
| Indium | +3 | Indiumnitrid (InN) | Indiumphosphid (InP) | Indiumarsenid (InAs) | Indiumantimonid (InSb) |
| Europium | +3 | Europium(III)-nitrid (EuN) | Europium(III)-phosphid (EuP) | ||
| Hafnium | +3/+4 | Hafniumnitrid (HfN/Hf3N4) | |||
| Tantal | +3/+5 | Tantalnitrid (TaN/Ta3N5) |
Boride, Carbide, Silicide, Auride und Polonide
| Element (Kation) | Oxidationszustand | Borid | Carbid | Silicid | Aurid | Polonid |
|---|---|---|---|---|---|---|
| Lithium | +1 | Lithiumcarbid (Li2C2) | Lithiumpolonid (Li2Po) | |||
| Beryllium | +2 | Bariumcarbid (BaC2) | ||||
| Bor | Borcarbid (B4C) | |||||
| Natrium | +1 | Natriumcarbid (Na2C2) | Natriumpolonid (Na2Po) | |||
| Magnesium | Magnesiumdiborid (MgB2) | |||||
| +2 | Magnesiumcarbid (Mg2C3) | Magnesiumsilicid (Mg2Si) | Magnesiumpolonid (MgPo) | |||
| Aluminium | Aluminiumdiborid (AlB2) Aluminiumdodecaborid (AlB12) | |||||
| +3 | Aluminiumcarbid (Al4C3) | |||||
| Silicium | +4 | Siliciumcarbid (SiC) | ||||
| Kalium | +1 | Kaliumcarbid (K2C2) | Kaliumpolonid (K2Po) | |||
| Calciummonosilicid (CaSi) Dicalciumsilicid (Ca2Si) Pentacalciumtrisilicid (Ca5Si3) | ||||||
| Calcium | +2 | Calciumborid (CaB6) | Calciumcarbid (CaC2) | Calciumdisilicid (CaSi2) | ||
| Titan | Titanborid (TiB2) | Titandisilicid (TiSi2) | ||||
| +4 | Titancarbid (TiC) | |||||
| Vanadium | +4 | Vanadiumcarbid (VC) | ||||
| Chrom | Chromcarbid (Cr3C2) | |||||
| +2 | Chromdisilicid (CrSi2) | |||||
| +3 | Chrom(III)-borid (CrB) | |||||
| Eisen | Eisencarbid (Fe3C) | |||||
| Cobalt | Dicobaltcarbid (Co2C) | |||||
| +2 | Cobaltdisilicid (CoSi2) | |||||
| Nickel | Dinickelborid (Ni2B) | Trinickelcarbid (Ni3C) | Dinickelsilicid (Ni2Si) | |||
| +2 | Nickeldisilicid (NiSi2) | |||||
| Kupfer | Pentakupfersilicid (Cu5Si) | |||||
| +1 | Kupfercarbid (Cu2C2) | |||||
| Rubidium | +1 | Rubidiumcarbid (Rb2C2) | ||||
| Strontium | +2 | Strontiumborid (SrB6) | Strontiumcarbid (SrC2) | |||
| Zirconium | Zirconiumdiborid (SrB6) | Zirconiumdisilicid (ZrSi2) | ||||
| +4 | Zirconiumcarbid (ZrC) | |||||
| Niob | Niobdiborid (NbB2) | |||||
| +4 | Niobcarbid (NbC) | |||||
| Molybdän | Molybdäncarbid (Mo2C) | Molybdändisilicid (MoSi2) | ||||
| Caesium | +1 | Caesiumaurid (CsAu) | ||||
| Barium | +2 | Bariumhexaborid (BaB6) | ||||
| Lanthan | +3 | Lanthanhexaborid (LaB6) | ||||
| Cer | +3 | Cerhexaborid (CeB6) | ||||
| Samarium | +3 | Samariumhexaborid (SmB6) | ||||
| Hafnium | Hafniumdiborid (HfB2) | |||||
| +4 | Hafniumcarbid (HfC) | |||||
| Tantal | Tantaldiborid (TaB2) | |||||
| +4 | Tantalcarbid (TaC) | |||||
| Wolfram | +4 | Wolframcarbid (WC) | Wolframdisilicid (WSi2) | |||
| Rhenium | Rheniumdiborid (ReB2) | |||||
| Platin | Platinsilicid (PtSi) | |||||
| Blei | +2 | Bleipolonid (PbPo) | ||||
| Uran | Urandiborid (UB2) | |||||
| +2 | Urandicarbid (UC2) Diurantricarbid (U2C3) | |||||
| +4 | Uran(IV)-carbid (UC) | |||||
| Plutonium | Plutoniumdiborid (PuB2) | |||||
| +3 | Plutoniumborid (PuB) |
Salze der Sauerstoffsäuren
Die Salze der Sauerstoffsäuren haben ein Anion, dass aus Sauerstoff und mindestens einem weiteren Element besteht.
Carbonate, Nitrate, Phosphate und Sulfate
| Element (Kation) | Oxidationszustand | Carbonat | Nitrat | Phosphat | Sulfat |
|---|---|---|---|---|---|
| Lithium | +1 | Lithiumcarbonat (Li2CO3) | Lithiumnitrat (LiNO3) | Lithiumphosphat (Li3PO4) | Lithiumsulfat (Li2SO4) |
| Beryllium | +2 | Berylliumcarbonat (BeCO3) | Berylliumnitrat (Be(NO3)2) | Berylliumphosphat (Be3(PO4)2) | Berylliumsulfat (BeSO4) |
| Bor | +3 | Borphosphat (BPO4) | |||
| Natrium | +1 | Natriumcarbonat (Na2CO3) | Natriumnitrat (NaNO3) | Natriumphosphat (Na3PO4) Natriumdiphosphat (Na4P2O7) Pentanatriumtriphosphat (Na5P3O10) | Natriumsulfat (Na2SO4) Natriumdisulfat (Na2S2O7) |
| Magnesium | +2 | Magnesiumcarbonat (MgCO3) | Magnesiumnitrat (Mg(NO3)2) | Magnesiumphosphat (Mg3(PO4)2) Magnesiumdiphosphat (Mg2P2O7) | Magnesiumsulfat (MgSO4) |
| Aluminium | +3 | Aluminiumnitrat (Al(NO3)3) | Aluminiumorthophosphat (AlPO4) Aluminiummetaphosphat (Al(PO3)3) | Aluminiumsulfat (Al2(SO4)3) | |
| Kalium | +1 | Kaliumcarbonat (K2CO3) | Kaliumnitrat (KNO3) | Kaliumphosphat (K3PO4) Kaliumdiphosphat (K4P2O7) Pentakaliumtriphosphat (K5P3O10) | Kaliumsulfat (K2SO4) Kaliumdisulfat (K2S2O7) |
| Calcium | +2 | Calciumcarbonat (CaCO3) | Calciumnitrat (Ca(NO3)2) | Calciumphosphat (Ca3(PO4)2) Calciumdiphosphat (Ca2P2O7) | Calciumsulfat (CaSO4) |
| Scandium | +3 | Scandiumnitrat (Sc(NO3)3) | Scandiumsulfat (Sc2(SO4)3) | ||
| Titan | +3 | Titan(III)-sulfat (Ti2(SO4)3) | |||
| +4 | Titannitrat (Ti(NO3)4) | ||||
| Vanadium | +3 | Vanadium(III)-sulfat (V2(SO4)3) | |||
| +4 | Vanadylsulfat (VOSO4) | ||||
| Chrom | +2 | Chrom(II)-sulfat (CrSO4) | |||
| +3 | Chrom(III)-nitrat (Cr(NO3)3) | Chrom(III)-phosphat (CrPO4) | Chrom(III)-sulfat (Cr2(SO4)3) | ||
| Mangan | +2 | Mangan(II)-carbonat (MnCO3) | Mangan(II)-nitrat (Mn(NO3)2) | Mangan(II)-phosphat (Mn3(PO4)2) | Mangan(II)-sulfat (MnSO4) |
| Eisen | +2 | Eisen(II)-carbonat (FeCO3) | Eisen(II)-nitrat (Fe(NO3)2) | Eisen(II)-phosphat (Fe3(PO4)2) | Eisen(II)-sulfat (FeSO4) |
| +3 | Eisen(III)-nitrat (Fe(NO3)3) | Eisen(III)-phosphat (FePO4) | Eisen(III)-sulfat (Fe2(SO4)3) | ||
| Cobalt | +2 | Cobalt(II)-carbonat (CoCO3) | Cobalt(II)-nitrat (Co(NO3)2) | Cobalt(II)-phosphat (Co3(PO4)2) | Cobalt(II)-sulfat (CoSO4) |
| +3 | Cobalt(III)-nitrat (Co(NO3)3) | Cobalt(III)-phosphat (CoPO4) | Cobalt(III)-sulfat (Co2(SO4)3) | ||
| Nickel | +2 | Nickel(II)-carbonat (NiCO3) | Nickel(II)-nitrat (Ni(NO3)2) | Nickel(II)-phosphat (Ni3(PO4)2) Nickeldiphosphat (Ni2P2O7) | Nickel(II)-sulfat (NiSO4) |
| Kupfer | +1 | Kupfer(I)-nitrat (CuNO3) | Kupfer(I)-sulfat (Cu2SO4) | ||
| +2 | Kupfer(II)-carbonat (CuCO3) | Kupfer(II)-nitrat (Cu(NO3)2) | Kupfer(II)-phosphat (Cu3(PO4)2) | Kupfer(II)-sulfat (CuSO4) | |
| Zink | +2 | Zinkcarbonat (ZnCO3) | Zinknitrat (Zn(NO3)2) | Zinkphosphat (Zn3(PO4)2) Zinkdiphosphat (Zn2P2O7) | Zinksulfat (ZnSO4) |
| Gallium | +3 | Galliumnitrat (Ga(NO3)3) | Galliumphosphat (GaPO4) | ||
| Rubidium | +1 | Rubidiumcarbonat (Rb2CO3) | Rubidiumnitrat (RbNO3) | Rubidiumphosphat (Rb3PO4) | Rubidiumsulfat (Rb2SO4) |
| Strontium | +2 | Strontiumcarbonat (SrCO3) | Strontiumnitrat (Sr(NO3)2) | Strontiumphosphat (Sr3(PO4)2) | Strontiumsulfat (SrSO4) |
| Yttrium | +3 | Yttriumnitrat (Y(NO3)3) | Yttriumphosphat (YPO4) | Yttriumsulfat (Y2(SO4)3) | |
| Zirconium | +4 | Zirconiumnitrat (Zr(NO3)4) | Zirconium(IV)-sulfat (Zr(SO4)2) | ||
| Rhodium | +3 | Rhodium(III)-nitrat (Rh(NO3)3) | Rhodium(III)-phosphat (RhPO4) | Rhodium(III)-sulfat (Rh2(SO4)3) | |
| Palladium | +2 | Palladium(II)-carbonat (PdCO3) | Palladium(II)-nitrat (Pd(NO3)2) | Palladium(II)-phosphat (Pd3(PO4)2) | Palladium(II)-sulfat (PdSO4) |
| Silber | +1 | Silbercarbonat (Ag2CO3) | Silbernitrat (AgNO3) | Silberphosphat (Ag3PO4) | Silbersulfat (Ag2SO4) |
| Cadmium | +2 | Cadmiumcarbonat (CdCO3) | Cadmiumnitrat (Cd(NO3)2) | Cadmiumphosphat (Cd3(PO4)2) | Cadmiumsulfat (CdSO4) |
| Indium | +3 | Indium(III)-nitrat (In(NO3)3) | Indium(III)-phosphat (InPO4) | Indium(III)-sulfat (In2(SO4)3) | |
| Zinn | +2 | Zinn(II)-carbonat (SnCO3) | Zinn(II)-nitrat (Sn(NO3)2) | Zinn(II)-phosphat (Sn3(PO4)2) Zinn(II)-diphosphat (Sn2P2O7) | Zinn(II)-sulfat (SnSO4) |
| +4 | Zinn(IV)-sulfat (Sn(SO4)2) | ||||
| Antimon | +3 | Antimon(III)-nitrat (Sb(NO3)3) | Antimon(III)-phosphat (SbPO4) | Antimon(III)-sulfat (Sb2(SO4)3) | |
| Caesium | +1 | Caesiumcarbonat (Cs2CO3) | Caesiumnitrat (CsNO3) | Caesiumphosphat (Cs3PO4) | Caesiumsulfat (Cs2SO4) |
| Barium | +2 | Bariumcarbonat (BaCO3) | Bariumnitrat (Ba(NO3)2) | Bariumphosphat (Ba3(PO4)2) | Bariumsulfat (BaSO4) |
| Lanthan | +3 | Lanthancarbonat (La2(CO3)3) | Lanthannitrat (La(NO3)3) | Lanthanphosphat (LaPO4) | Lanthansulfat (La2(SO4)3) |
| Cer | +3 | Cer(III)-carbonat (Ce2(CO3)3) | Cer(III)-nitrat (Ce(NO3)3) | Cer(III)-phosphat (CePO4) | Cer(III)-sulfat (Ce2(SO4)3) |
| +4 | Cer(IV)-sulfat (Ce(SO4)2) | ||||
| Praseodym | +3 | Praseodym(III)-nitrat (Pr(NO3)3) | Praseodym(III)-phosphat (PrPO4) | Praseodym(III)-sulfat (Pr2(SO4)3) | |
| Europium | +2 | Europium(II)-carbonat (EuCO3) | Europium(II)-phosphat (Eu3[PO4]2) | Europium(II)-sulfat (EuSO4) | |
| +3 | Europium(III)-nitrat (Eu(NO3)3) | Europium(III)-sulfat (Eu2(SO4)3) | |||
| Thorium | +4 | Thoriumnitrat (Th(NO3)4) | |||
| Uran | +4 | Uran(IV)-sulfat (U(SO4)2) | |||
| +6 | Uranylcarbonat (UO2CO3) | Uranylnitrat (UO2(NO3)2) | Uranylsulfat (UO2SO4) |
Hydrogencarbonate, Hydrogenphosphate, Dihydrogenphosphate und Hydrogensulfate
| Element (Kation) | Oxidationszustand | Hydrogencarbonat | Hydrogenphosphat | Dihydrogenphosphat | Hydrogensulfat |
|---|---|---|---|---|---|
| Natrium | +1 | Natriumhydrogencarbonat (NaHCO3) | Dinatriumhydrogenphosphat (Na2HPO4) | Natriumdihydrogenphosphat (NaH2PO4) Dinatriumdihydrogendiphosphat (Na2H2P2O7) | Natriumhydrogensulfat (NaHSO4) |
| Magnesium | +2 | Magnesiumhydrogencarbonat (Mg(HCO3)2) | Magnesiumhydrogenphosphat (MgHPO4) | Magnesiumdihydrogenphosphat (Mg(H2PO4)2) | |
| Kalium | +1 | Kaliumhydrogencarbonat (KHCO3) | Dikaliumhydrogenphosphat (K2HPO4) | Kaliumdihydrogenphosphat (KH2PO4) Dikaliumdihydrogendiphosphat (K2H2P2O7) | Kaliumhydrogensulfat (KHSO4) |
| Calcium | +2 | Calciumhydrogencarbonat (Ca(HCO3)2) | Calciumhydrogenphosphat (CaHPO4) | Calciumdihydrogenphosphat (Ca(H2PO4)2) |
Nitrite, Sulfite, Hydrogensulfite, Thiosulfate und Persulfate
| Element (Kation) | Oxidationszustand | Nitrit | Sulfit | Hydrogensulfit | Thiosulfat | Persulfat |
|---|---|---|---|---|---|---|
| Lithium | +1 | Lithiumnitrit (LiNO2) | Lithiumsulfit (Li2SO3) | Lithiumhydrogensulfit (LiHSO3) | Lithiumthiosulfat (Li2S2O3) | Lithiumpersulfat (Li2S2O8) |
| Beryllium | +2 | Berylliumnitrit (Be(NO2)2) | Berylliumsulfit (BeSO3) | Berylliumhydrogensulfit (Be(HSO3)2) | Berylliumthiosulfat (BeS2O3) | |
| Natrium | +1 | Natriumnitrit (NaNO2) | Natriumsulfit (Na2SO3) | Natriumhydrogensulfit (NaHSO3) | Natriumthiosulfat (Na2S2O3) | Natriumpersulfat (Na2S2O8) |
| Magnesium | +2 | Magnesiumnitrit (Mg(NO2)2) | Magnesiumsulfit (MgSO3) | Magnesiumhydrogensulfit (Mg(HSO3)2) | Magnesiumthiosulfat (MgS2O3) | |
| Kalium | +1 | Kaliumnitrit (KNO2) | Kaliumsulfit (K2SO3) | Kaliumhydrogensulfit (KHSO3) | Kaliumthiosulfat (K2S2O3) | Kaliumpersulfat (K2S2O8) |
| Calcium | +2 | Calciumnitrit (Ca(NO2)2) | Calciumsulfit (CaSO3) | Calciumhydrogensulfit (Ca(HSO3)2) | Calciumthiosulfat (CaS2O3) | |
| Silber | +1 | Silbernitrit (AgNO2) | Silbersulfit (Ag2SO3) | Silberhydrogensulfit (AgHSO3) | ||
| Caesium | +1 | Caesiumthiosulfat (Cs2[S2O3]) |
Chlorate, Bromate und Iodate
| Element (Kation) | Oxidationszustand | Chlorat | Bromat | Iodat |
|---|---|---|---|---|
| Lithium | +1 | Lithiumchlorat (LiClO3) | Lithiumbromat (LiBrO3) | Lithiumiodat (LiIO3) |
| Beryllium | +2 | Berylliumchlorat (Be(ClO3)2) | Berylliumbromat (Be(BrO3)2) | Berylliumiodat (Be(IO3)2) |
| Natrium | +1 | Natriumchlorat (NaClO3) | Natriumbromat (NaBrO3) | Natriumiodat (NaIO3) |
| Magnesium | +2 | Magnesiumchlorat (Mg(ClO3)2) | Magnesiumbromat (Mg(BrO3)2) | Magnesiumiodat (Mg(IO3)2) |
| Kalium | +1 | Kaliumchlorat (KClO3) | Kaliumbromat (KBrO3) | Kaliumiodat (KIO3) |
| Calcium | +2 | Calciumchlorat (Ca(ClO3)2) | Calciumbromat (Ca(BrO3)2) | Calciumiodat (Ca(IO3)2) |
| Zink | +2 | Zinkchlorat (Zn(ClO3)2) | Zinkbromat (Zn(BrO3)2) | Zinkiodat (Zn(IO3)2) |
| Rubidium | +1 | Rubidiumchlorat (RbClO3) | Rubidiumbromat (RbBrO3) | Rubidiumiodat (RbIO3) |
| Strontium | +2 | Strontiumchlorat (Sr(ClO3)2) | Strontiumbromat (Sr(BrO3)2) | Strontiumiodat (Sr(IO3)2) |
| Silber | +1 | Silberchlorat (AgClO3) | Silberbromat (AgBrO3) | Silberiodat (AgIO3) |
| Caesium | +1 | Caesiumchlorat (CsClO3) | Caesiumbromat (CsBrO3) | Caesiumiodat (CsIO3) |
| Barium | +2 | Bariumchlorat (Ba(ClO3)2) | Bariumbromat (Ba(BrO3)2) | Bariumiodat (Ba(IO3)2) |
Perchlorate, Perbromate und Periodate
| Element (Kation) | Oxidationszustand | Perchlorat | Perbromat | Periodat |
|---|---|---|---|---|
| Lithium | +1 | Lithiumperchlorat (LiClO4) | Lithiumperbromat (LiBrO4) | Lithiumperiodat (LiIO4) |
| Beryllium | +2 | Berylliumperchlorat (Be(ClO4)2) | Berylliumperbromat (Be(BrO4)2) | Berylliumperiodat (Be(IO4)2) |
| Natrium | +1 | Natriumperchlorat (NaClO4) | Natriumperbromat (NaBrO4) | Natriumperiodat (NaIO4) |
| Magnesium | +2 | Magnesiumperchlorat (Mg(ClO4)2) | Magnesiumperbromat (Mg(BrO4)2) | Magnesiumperiodat (Mg(IO4)2) |
| Kalium | +1 | Kaliumperchlorat (KClO4) | Kaliumperbromat (KBrO4) | Kaliumperiodat (KIO4) |
| Calcium | +2 | Calciumperchlorat (Ca(ClO4)2) | Calciumperbromat (Ca(BrO4)2) | Calciumperiodat (Ca(IO4)2) |
| Rubidium | +1 | Rubidiumperchlorat (RbClO4) | Rubidiumperbromat (RbBrO4) | Rubidiumperiodat (RbIO4) |
| Strontium | +2 | Strontiumperchlorat (Sr(ClO4)2) | Strontiumperbromat (Sr(BrO4)2) | Strontiumperiodat (Sr(IO4)2) |
| Silber | +1 | Silberperchlorat (AgClO4) | Silberperbromat (AgBrO4) | Silberperiodat (AgIO4) |
| Caesium | +1 | Caesiumperchlorat (CsClO4) | Caesiumperbromat (CsBrO4) | Caesiumperiodat (CsIO4) |
| Barium | +2 | Bariumperchlorat (Ba(ClO4)2) | Bariumperbromat (Ba(BrO4)2) | Bariumperiodat (Ba(IO4)2) |
Hypochlorite, Hypobromite und Hypoiodite
| Element (Kation) | Oxidationszustand | Hypochlorit | Hypobromit | Hypoiodit |
|---|---|---|---|---|
| Lithium | +1 | Lithiumhypochlorit (LiClO) | Lithiumhypobromit (LiBrO) | Lithiumhypoiodit (LiIO) |
| Beryllium | +2 | Berylliumhypochlorit (Be(ClO)2) | Berylliumhypobromit (Be(BrO)2) | Berylliumhypoiodit (Be(IO)2) |
| Natrium | +1 | Natriumhypochlorit (NaClO) | Natriumhypobromit (NaBrO) | Natriumhypoiodit (NaIO) |
| Magnesium | +2 | Magnesiumhypochlorit (Mg(ClO)2) | Magnesiumhypobromit (Mg(BrO)2) | Magnesiumhypoiodit (Mg(IO)2) |
| Kalium | +1 | Kaliumhypochlorit (KClO) | Kaliumhypobromit (KBrO) | Kaliumhypoiodit (KIO) |
| Calcium | +2 | Calciumhypochlorit (Ca(ClO)2) | Calciumhypobromit (Ca(BrO)2) | Calciumhypoiodit (Ca(IO)2) |
Borate, Aluminate und Silicate
| Element (Kation) | Oxidationszustand | Borat | Aluminat | Silicat |
|---|---|---|---|---|
| Lithium | +1 | Lithiummetaborat (LiBO2) Lithiumtriborat (LiB3O5) Lithiumtetraborat (Li2B4O7) | Lithiumaluminat (LiAlO2) | |
| Beryllium | +2 | Berylliumborat (Be3(BO3)2) | ||
| Natrium | +1 | Natriummetaborat (NaBO2) Natriumtetraborat (Na2B4O7) Natriumpentaborat (NaB5O8) | Natriummetaaluminat (NaAlO2) | Natriumorthosilicat (Na4SiO4) Dinatriummetasilicat (Na2SiO3) Dinatriumdisilicat (Na2Si2O5) Dinatriumtrisilicat (Na2Si3O7) |
| Magnesium | +2 | Magnesiumborat (Mg3(BO3)2) | Magnesiumorthosilicat (Mg2SiO4) Magnesiummetasilicat (MgSiO3) Magnesiumtrisilicat (Mg2Si3O8) | |
| Kalium | +1 | Kaliummetaborat (KBO2) Dikaliumtetraborat (K2B4O7) | Kaliumsilicat (K2Si2O5) | |
| Calcium | +2 | Calciumborat (Ca3(BO3)2) | Monocalciumaluminat (CaAl2O4) Tricalciumaluminat (Ca3Al2O6) | Calciumsilicat (CaSiO3) |
| Strontium | +2 | Strontiumaluminat (SrAl2O4) | ||
| Zirconium | +4 | Zirconiumsilicat (ZrSiO4) | ||
| Barium | +2 | Bariumborat (Ba(BO2)2) | ||
| Europium | +2 | Europium(II)-borat (Eu3[BO3]2) | Europium(II)-silicat (Eu2[SiO4]) | |
| +3 | Europium(III)-borat (Eu[BO3]) | |||
| Hafnium | +4 | Hafniumsilicat (HfSiO4) |
Titanate, Vanadate, Chromate, Manganate und Permanganate
| Element (Kation) | Oxidationszustand | Titanat | Vanadat | Chromat | Manganat | Permanganat |
|---|---|---|---|---|---|---|
| Lithium | +1 | Lithiummetatitanat (Li2TiO3) | Lithiumchromat (Li2CrO4) Lithiumdichromat (Li2Cr2O7) | Lithiummanganat (Li2MnO4) | Lithiumpermanganat (LiMnO4) | |
| Beryllium | +2 | Berylliumtitanat (BeTiO3) | Berylliumchromat (BeCrO4) | Berylliumpermanganat (Be(MnO4)2) | ||
| Natrium | +1 | Natriummetatitanat (Na2Ti3O7) | Natriumorthovanadat (Na3VO4) Natriummetavanadat (NaVO3) | Natriumchromat (Na2CrO4) Natriumdichromat (Na2Cr2O7) | Natriummanganat (Na2MnO4) | Natriumpermanganat (NaMnO4) |
| Magnesium | +2 | Magnesiumtitanat (MgTiO3) | Magnesiumchromat (MgCrO4) | Magnesiumpermanganat (Mg(MnO4)2) | ||
| Kalium | +1 | Kaliummetatitanat (K2TiO3) | Kaliummetavanadat (KVO3) | Kaliumchromat (K2CrO4) Kaliumdichromat (K2Cr2O7) | Kaliummanganat (K2MnO4) | Kaliumpermanganat (KMnO4) |
| Calcium | +2 | Calciumtitanat (CaTiO3) | Calciumchromat (CaCrO4) | Calciumpermanganat (Ca(MnO4)2) | ||
| Chrom | +3 | Chrom(III)-chromat (Cr2(CrO4)3) | ||||
| Eisen | +3 | Eisen(III)-chromat (Fe2(CrO4)3) | ||||
| Nickel | +2 | Nickel(II)-titanat (NiTiO3) | ||||
| Zink | +2 | Zinkchromat (ZnCrO4) | ||||
| Rubidium | +1 | Rubidiummetatitanat (Rb2TiO3) | Rubidiumchromat (Rb2CrO4) Rubidiumdichromat (Rb2Cr2O7) | Rubidiummanganat (Rb2MnO4) | Rubidiumpermanganat (RbMnO4) | |
| Strontium | +2 | Strontiumtitanat (SrTiO3) | Strontiumchromat (SrCrO4) | Strontiumpermanganat (Sr(MnO4)2) | ||
| Yttrium | +3 | Yttriumorthovanadat (YVO4) | ||||
| Silber | +1 | Silberchromat (Ag2CrO4) Silberdichromat (Ag2Cr2O7) | Silbermanganat (Ag2MnO4) | Silberpermanganat (AgMnO4) | ||
| Caesium | +1 | Caesiummetatitanat (Cs2TiO3) | Caesiumchromat (Cs2CrO4) Caesiumdichromat (Cs2Cr2O7) | Caesiummanganat (Cs2MnO4) | Caesiumpermanganat (BaMnO4) | |
| Barium | +2 | Bariumtitanat (BaTiO3) | Bariummanganat (BaMnO4) | Bariumpermanganat (Ba(MnO4)2) | ||
| Europium | +2 | Europium(II)-titanat (EuTiO3) | ||||
| Blei | +2 | Bleititanat (PbTiO3) | Blei(II)-chromat (PbCrO4) | |||
| Bismut | +3 | Bismutvanadat (BiVO4) |
Ferrate, Arsenate, Arsenite, Selenate und Selenite
| Element (Kation) | Oxidationszustand | Ferrat | Arsenat | Arsenit | Selenat | Selenit |
|---|---|---|---|---|---|---|
| Lithium | +1 | Lithiumferrat (Li2FeO4) | Lithiumarsenat (Li3AsO4) | Lithiumarsenit (LiAsO2) | Lithiumselenat (Li2SeO4) | Lithiumselenit (Li2SeO3) |
| Beryllium | +2 | Berylliumferrat (BeFeO4) | Berylliumarsenat (Be3(AsO4)2) | Berylliumselenat (BeSeO4) | Berylliumselenit (BeSeO3) | |
| Natrium | +1 | Natriumferrat (Na2FeO4) | Natriumarsenat (Na3AsO4) Dinatriumhydrogenarsenat (Na2HAsO4) Natriumdihydrogenarsenat (NaH2AsO4) | Natriumarsenit (NaAsO2) | Natriumselenat (Na2SeO4) | Natriumselenit (Na2SeO3) |
| Magnesium | +2 | Magnesiumferrat (MgFeO4) | Magnesiumarsenat (Mg3(AsO4)2) | Magnesiumselenat (MgSeO4) | Magnesiumselenit (MgSeO3) | |
| Kalium | +1 | Kaliumferrat (K2FeO4) | Kaliumarsenat (K3AsO4) | Kaliummetaarsenit (KAsO2) | Kaliumselenat (K2SeO4) | Kaliumselenit (K2SeO3) |
| Calcium | +2 | Calciumferrat (CaFeO4) | Calciumarsenat (Ca3(AsO4)2) | Calciumselenat (CaSeO4) | Calciumselenit (CaSeO3) | |
| Kupfer | +2 | Kupfer(II)-arsenat (Cu3(AsO4)2) | Kupfer(II)-arsenit (CuHAsO3) | Kupferselenit (CuSeO3) | ||
| Rubidium | +1 | Rubidiumferrat (Rb2FeO4) | Rubidiumarsenat (Rb3AsO4) | Rubidiumarsenit (RbAsO2) | Rubidiumselenat (Rb2SeO4) | Rubidiumselenit (Rb2SeO3) |
| Strontium | +2 | Strontiumferrat (SrFeO4) | Strontiumarsenat (Sr3(AsO4)2) | Strontiumselenat (SrSeO4) | Strontiumselenit (SrSeO3) | |
| Silber | +1 | Silberarsenat (Ag3AsO4) | Silberselenit (Ag2SeO3) | |||
| Caesium | +1 | Caesiumferrat(Cs2FeO4) | Caesiumarsenat (Cs3AsO4) | Caesiumarsenit (CsAsO2) | Caesiumselenat (Cs2SeO4) | Caesiumselenit (Cs2SeO3) |
| Barium | +2 | Bariumferrat (BaFeO4) | Bariumarsenat (Ba3(AsO4)2) | Bariumselenat (BaSeO4) | Bariumselenit (BaSeO3) | |
| Gold | +3 | Gold(III)-selenat (Au2(SeO4)3) | ||||
| Blei | +2 | Blei(II)-arsenat (Pb3(AsO4)2) Bleihydrogenarsenat (PbHAsO4) | Blei(II)-selenat (PbSeO4) |
Molybdate, Tellurite, Tellurate, Tantalate, Perxenate und Wolframate
| Element (Kation) | Oxidationszustand | Molybdat | Tellurit | Tellurat | Tantalat | Perxenat | Wolframat |
|---|---|---|---|---|---|---|---|
| Lithium | +1 | Lithiummolybdat (Li2MoO4) | Lithiumtellurat (Li2TeO4) | Lithiumtantalat (LiTaO3) | Lithiumwolframat (Li2WO4) | ||
| Beryllium | +2 | Berylliummolybdat (BeMoO4) | Berylliumtellurat (BaTeO4) | Berylliumwolframat (BaWO4) | |||
| Natrium | +1 | Natriummolybdat (Na2MoO4) | Natriumtellurit (Na2TeO3) | Natriumtellurat (Na2TeO4) | Natriumtantalat (NaTaO3) | Natriumperxenat (Na4XeO6) | Natriumwolframat (Na2WO4) |
| Magnesium | +2 | Magnesiummolybdat (MgMoO4) | Magnesiumtellurat (MgTeO4) | Magnesiumwolframat (MgWO4) | |||
| Kalium | +1 | Kaliummolybdat (K2MoO4) | Kaliumtellurit (K2TeO3) | Kaliumtellurat (K2TeO4) | Kaliumtantalat (KTaO3) | Kaliumperxenat (K4XeO6) | Kaliumwolframat (K2WO4) |
| Calcium | +2 | Calciummolybdat (CaMoO4) | Calciumtellurit (CaTeO3) | Calciumtellurat (CaTeO4) | Calciumwolframat (CaWO4) | ||
| Rubidium | +1 | Rubidiummolybdat (Rb2MoO4) | Rubidiumtellurat (Rb2TeO4) | Rubidiumtantalat (RbTaO3) | Rubidiumwolframat (Rb2WO4) | ||
| Strontium | +2 | Strontiummolybdat (SrMoO4) | Strontiumtellurit (SrTeO3) | Strontiumtellurat (SrTeO4) | Strontiumwolframat (SrWO4) | ||
| Caesium | +1 | Caesiummolybdat (Cs2MoO4) | Caesiumtellurat (Cs2TeO4) | Caesiumtantalat (CsTaO3) | Caesiumwolframat (Cs2WO4) | ||
| Barium | +2 | Bariummolybdat (BaMoO4) | Bariumtellurit (BaTeO3) | Bariumtellurat (BaTeO4) | Bariumperxenat (Ba2XeO6) | Bariumwolframat (BaWO4) |
Liste von anorganischen Basen
Hydroxide, Hydrogensulfide und Amide
Hydroxide enthalten Hydroxidionen als negativ geladene Ionen. Hydrogensulfide sind Salze von Schwefelwasserstoff. Ionische Amide sind von Ammoniak abgeleitet.
| Element (Kation) | Oxidationszustand | Hydroxid | Hydrogensulfid | Amid |
|---|---|---|---|---|
| Lithium | +1 | Lithiumhydroxid (LiOH) | Lithiumhydrogensulfid (LiHS) | Lithiumamid (LiNH2) |
| Beryllium | +2 | Berylliumhydroxid (Be(OH)2) | Berylliumhydrogensulfid (Be(HS)2) | |
| Natrium | +1 | Natriumhydroxid (NaOH) | Natriumhydrogensulfid (NaHS) | Natriumamid (NaNH2) |
| Magnesium | +2 | Magnesiumhydroxid (Mg(OH)2) | Magnesiumhydrogensulfid (Mg(HS)2) | |
| Aluminium | +3 | Aluminiumhydroxid (Al(OH)3) | ||
| Kalium | +1 | Kaliumhydroxid (KOH) | Kaliumhydrogensulfid (KHS) | Kaliumamid (KNH2) |
| Calcium | +2 | Calciumhydroxid (Ca(OH)2) | Calciumhydrogensulfid (Ca(HS)2) | |
| Mangan | +2 | Mangan(II)-hydroxid (Mn(OH)2) | ||
| Eisen | +2 | Eisen(II)-hydroxid (Fe(OH)2) | ||
| +3 | Eisen(III)-hydroxidoxid (FeO(OH)) | |||
| Cobalt | +2 | Cobalt(II)-hydroxid (Co(OH)2) | ||
| +3 | Cobalt(III)-hydroxid (Co(OH)3) | |||
| Nickel | +2 | Nickel(II)-hydroxid (Ni(OH)2) | ||
| Kupfer | +2 | Kupfer(II)-hydroxid (Cu(OH)2) | ||
| Zink | +2 | Zinkhydroxid (Zn(OH)2) | Zinkamid (Zn(NH2)2) | |
| Gallium | +3 | Galliumhydroxid (Ga(OH)3) | ||
| Rubidium | +1 | Rubidiumhydroxid (RbOH) | Rubidiumamid (RbNH2) | |
| Strontium | +2 | Strontiumhydroxid (Sr(OH)2) | ||
| Zirconium | +4 | Zirconium(IV)-hydroxid (Zr(OH)4) | ||
| Silber | +1 | Silberhydroxid (AgOH) | ||
| Cadmium | +2 | Cadmiumhydroxid (Cd(OH)2) | Cadmiumamid (Cd(NH2)2) | |
| Indium | +3 | Indium(III)-hydroxid (In(OH)3) | ||
| Zinn | +2 | Zinn(II)-hydroxid (Sn(OH)2) | ||
| Caesium | +1 | Caesiumhydroxid (CsOH) | ||
| Barium | +2 | Bariumhydroxid (Ba(OH)2) | ||
| Lanthan | +3 | Lanthanhydroxid (La(OH)3) | ||
| Cer | +3 | Cer(III)-hydroxid (Ce(OH)3) | ||
| Gold | +3 | Gold(III)-hydroxid (Au(OH)3) | ||
| Quecksilber | +2 | Quecksilber(II)-hydroxid (Hg(OH)2) | ||
| Thallium | +1 | Thallium(I)-hydroxid (TlOH) | ||
| +3 | Thallium(III)-hydroxid (Tl(OH)3) | |||
| Blei | +2 | Blei(II)-hydroxid (Pb(OH)2) | ||
| +4 | Blei(IV)-hydroxid (Pb(OH)4) | |||
| Uran | +6 | Uranylhydroxid (UO2(OH)2) |
Liste von anorganischen Säuren
Umgangssprachliche Namen (Trivialnamen)
Die folgende Tabelle listet Trivialnamen mit den entsprechenden systematischen Namen (IUPAC-Namen) bekannter anorganischer Salze alphabetisch auf.
| Trivialname | IUPAC-Name | Strukturformel | Formel | Kristallstruktur | Kristallsystem |
|---|---|---|---|---|---|
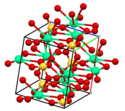
| Alundum | Aluminiumoxid | Al2O3 |  | trigonal | |
| Ammoniaksalpeter | Ammoniumnitrat |  | NH4NO3 | orthorhombisch | |
| Antichlor (siehe Fixiersalz) | |||||
| Arsenik | Arsen(III)-oxid | As2O3 |  | monoklin | |
| Ätzkali | Kaliumhydroxid | KOH | rhomboedrisch | ||
| Ätzkalk (siehe Gebrannter Kalk) | |||||
| Ätznatron | Natriumhydroxid | NaOH | orthorhombisch | ||
| Ätzsoda (siehe Ätznatron) | |||||
| Backnatron (siehe Natron) | |||||
| Backsoda (siehe Natron) | |||||
| Barytsalpeter | Bariumnitrat |  | Ba(NO3)2 | kubisch | |
| Beryllerde | Berylliumoxid | BeO | 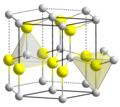 | hexagonal | |
| Bittererde (siehe Magnesia) | |||||
| Bittersalzerde (siehe Magnesia) | |||||
| Blaukupfer | Kupfer(II)-hydroxid | Cu(OH)2 |  | ||
| Blaustein | Kupfersulfat | CuSO4 | 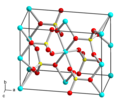 | orthorhombisch | |
| Bleiglätte | Blei(II)-oxid | PbO |  | tetragonal | |
| Bleimennige | Blei(II,IV)-oxid | Pb3O4 | 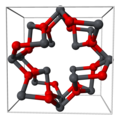 | tetragonal | |
| Branntkalk (siehe Gebrannter Kalk) | |||||
| Braunstein | Mangan(IV)-oxid | MnO2 |  | tetragonal | |
| Bullrichsalz (siehe Natron) | |||||
| Calciniertes Soda (siehe Waschsoda) | |||||
| Carborund (siehe Karborund) | |||||
| Cobaltschwarz | Cobalt(II,III)-oxid | Co3O4 |  | kubisch | |
| Doppeltkohlensaures Natron (siehe Natron) | |||||
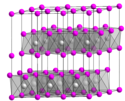
| Eisenmennige | Eisen(III)-oxid | Fe2O3 |  | rhomboedrisch | |
| Eisenvitriol | Eisen(II)-sulfat | 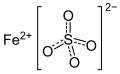 | FeSO4 | orthorhombisch | |
| Eisen(III)-sulfat | |||||
| Fixiersalz | Natriumthiosulfat |  | Na2S2O3 | monoklin | |
| Freikalk (siehe Gebrannter Kalk) | |||||
| Gebrannter Kalk | Calciumoxid | CaO | (c) Goran tek-en, CC BY-SA 4.0 | kubisch | |
| Gebranntes Magnesia (siehe Gebrannter Kalk) | |||||
| Gelber Arsenik (siehe Gelbes Schwefelarsen) | |||||
| Gelbes Schwefelarsen | Arsen(III)-sulfid | As2S3 |  | ||
| Gelöschter Kalk | Calciumhydroxid | Ca(OH)2 |  | hexagonal | |
| Gips | Calciumsulfat |  | CaSO4 |  | orthorhombisch |
| Hammerschlag | Eisen(II,III)-oxid | Fe3O4 |  | ||
| Hirschhornsalz | Ammoniumhydrogencarbonat |  | NH4HCO3 | ||
| Höllenstein | Silbernitrat |  | AgNO3 | orthorhombisch | |
| Hornsalz | Blei(II)-chlorid | PbCl2 |  | orthorhombisch | |
| Kalihydrat (siehe Ätzkali) | |||||
| Kalisalpeter | Kaliumnitrat |  | KNO3 | orthorhombisch | |
| Kalkerde (siehe Gebrannter Kalk) | |||||
| Kalkhydrat (siehe Gelöschter Kalk) | |||||
| Kalksalpeter | Calciumnitrat |  | Ca(NO3)2 | kubisch | |
| Karborund | Siliciumcarbid | SiC | kubisch | ||
| Kaustisches Soda (siehe Ätznatron) | |||||
| Knallsilber | Silbernitrid | Ag3N |  | kubisch flächenzentriert | |
| Kochsalz | Natriumchlorid | NaCl | (c) Goran tek-en, CC BY-SA 4.0 | kubisch | |
| Kohlensaurer Kalk | Calciumcarbonat |  | CaCO3 | trigonal | |
| Kohlensaures Kalium (siehe Pottasche) | |||||
| Kohlensaures Natron (siehe Waschsoda) | |||||
| Korund (siehe Alundum) | |||||
| Kupferasche | Kupfer(II)-oxid | CuO |  | monoklin | |
| Kupfervitriol (siehe Blaustein) | |||||
| Löschkalk (siehe Gelöschter Kalk) | |||||
| Lötstein | Ammoniumchlorid |  | NH4Cl | kubisch | |
| Magnesia | Magnesiumoxid | MgO | (c) Goran tek-en, CC BY-SA 4.0 | kubisch | |
| Magnesiumcarbonat | 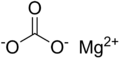 | MgCO3 | trigonal | ||
| Mauersalpeter (siehe Kalksalpeter) | |||||
| Mennige (siehe Bleimennige) | |||||
| Natron | Natriumhydrogencarbonat |  | NaHCO3 | monoklin | |
| Natronhydrat (siehe Ätznatron) | |||||
| Natronsalpeter | Natriumnitrat | 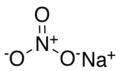 | NaNO3 | 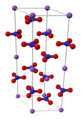 | trigonal |
| Pottasche | Kaliumcarbonat |  | K2CO3 | monoklin | |
| Schwefeleisen | Eisen(II)-sulfid | FeS |  | hexagonal dichteste Kugelpackung | |
| Schwefelnickel | Nickel(II)-sulfid | NiS |  | hexagonal dichteste Kugelpackung | |
| Schwefelsaures Natron | Natriumsulfat |  | Na2SO4 | orthorhombisch | |
| Schwefelsilber | Silbersulfid | Ag2S |  | monoklin | |
| Silbersalpeter (siehe Höllenstein) | |||||
| Tafelsalz (siehe Natron) | |||||
| Sublimat | Quecksilber(II)-chlorid | HgCl2 | |||
| Sulfigran | Natriumsulfid | Na2S |  | kubisch | |
| Talkerde (siehe Magnesia) | |||||
| Tonerde (siehe Alundum) | |||||
| Ungelöschter Kalk (siehe Gebrannter Kalk) | |||||
| Waschsoda | Natriumcarbonat | 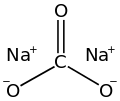 | Na2CO3 | monoklin | |
| Zementit | Eisencarbid | Fe3C |  | orthorhombisch | |
| Zinkvitriol | Zinksulfat |  | ZnSO4 | ||
| Zinkweiß | Zinkoxid | ZnO |  | hexagonal | |
| Zinnasche | Zinn(IV)-oxid | SnO2 |  | tetragonal | |
| Zinnober | Quecksilber(II)-sulfid | HgS |  | trigonal | |
| Zinnweiß (siehe Zinnasche) |
Auf dieser Seite verwendete Medien
Crystal structure of CaF2 with coordination polyhedra
Elementarzelle (Einheitszelle) von Hämatit, chemisch Eisen(III)-oxid
Chemical structure of silver nitrate.
Ball-and-stick model of a layer in the crystal structure of claudetite I, a high-temperature, monoclinic form of arsenic trioxide, As2O3.
X-ray crystallographic data from F. Pertlik (March 1978). "Verfeinerung der Kristallstruktur des Minerals Claudetit, As2O3 (“Claudetit I”)". Monatsh. Chem. 109 (2): 277-282. DOI:10.1007/BF00906344..
Model constructed in CrystalMaker 8.1.
Image generated in Accelrys DS Visualizer.Autor/Urheber: Orci, Lizenz: CC BY-SA 3.0
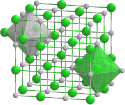
Kristallstruktur von de:Cadmiumiodid
Chemical structure of potassium carbonate
Ball-and-stick model of the unit cell of sodium nitrate, NaNO3. The structure has been reported several times, for example in Acta Cryst. B (1972) 28, 2700-2702 (data available from the CSD).
(c) Goran tek-en, CC BY-SA 4.0
Crystal structure of NaCl with coordination polyhedra
Crystal structure of ZnS (wurtzite) with coordination polyhedra
Chemical structure of calcium carbonate
Structural formula of calcium sulfate
Autor/Urheber: Orci, Lizenz: CC BY-SA 3.0
Kristallstruktur von Zementit
Iron(II) sulfate, structural formula
Zinksulfat
Ball-and-stick model of the unit cell of rhenium trioxide, ReO3
chemical structure of calcium nitrate
2D model of en:Ammonium bicarbonate.
| Ich, der Urheberrechtsinhaber dieses Werkes, veröffentliche es als gemeinfrei. Dies gilt weltweit. In manchen Staaten könnte dies rechtlich nicht möglich sein. Sofern dies der Fall ist: Ich gewähre jedem das bedingungslose Recht, dieses Werk für jedweden Zweck zu nutzen, es sei denn, Bedingungen sind gesetzlich erforderlich. |
Autor/Urheber: David Schrupp, Lizenz: CC BY-SA 2.0 de
Einheitszelle von Magnetit, Sauerstoff (grau), divalentes Eisen (grün), trivalentes Eisen (blau), Eisenion in Oktaederlücke (hellblauer Oktaeder), Eisenion in Tetraederlücke (grauer Tetraeder).
Structure of Potassium nitrate
Diagram of sodium carbonate drawn in ChemDraw, vectorized in Inkscape, optimized with Python scripts.
Autor/Urheber: Orci, Lizenz: CC BY-SA 3.0
Kristallstruktur von de:Nickelarsenid
Ball-and-stick model of the unit cell of cobalt(II,III) oxide, Co3O4.
Colour code:
- Cobalt(II), CoII: lighter blue
- Cobalt(III), CoIII: darker blue
- Oxygen, O: red
Crystal structure from J. Magn. Magn. Mater. (2006) 300, 300–305
Image generated in CrystalMaker 8.2.Ball-and-stick diagram of part of the crystal structure of the alpha polymorph of mercury sulfide, α-HgS. It is found in nature as the mineral cinnabar.
Colour code:
- Mercury, Hg: grey
- Sulfur, S: yellow
Crystal structure from Z. Krist., Supplement Issue (1999) 16, 95–95.
Image generated in CrystalMaker 8.2.Autor/Urheber: Orci, Lizenz: CC BY-SA 3.0
Kristallstruktur von de:Kupfer(II)-oxid
α-Silver(I) sulfide with the unit cell, monoclinic, P21/n, 4 molecules in the cell. According to "Silver sulfide (Ag2S) crystal structure" by Springer Link.
Chemical structure of ammonium chloride
Autor/Urheber: Orci, Lizenz: CC BY-SA 3.0
Kristallstruktur von de:Blei(II)-chlorid
chemical structure of magnesium carbonate
Autor/Urheber: Mrgreen71, Lizenz: CC BY-SA 3.0
Structure of mercury(II)chloride
Autor/Urheber: Orci, Lizenz: CC BY-SA 3.0
Kristallstruktur von Blei(II)-oxid
Sodium sulfate, structural formula
Autor/Urheber: Solid State, Lizenz: CC BY-SA 3.0
Kristallstruktur von Rutil (Titan(IV)-oxid, TiO2). Kristallographische Daten: https://dx.doi.org/10.1107/S0365110X56001388
Autor/Urheber: Andif1, Lizenz: CC BY-SA 4.0
Unit cell of anhydrous copper(II) sulfate. Created using Diamond 4. Data from Kokkoros, P.A.; Rentzeperis, P.J. The crystal structure of the anhydrous sulfates of copper and zinc Acta Crystallographica (1,1948-23,1967), 11, 361-364 (1958)
Kristallstruktur von Korund mit Blick entlang der b-Achse ([010])
Sodium thiosulfate, structural formula
chemical structure of barium nitrate